- WHAT WE DO
Additional Services

- Industries

Case Study: Multilingual Retail Marketing
New AI Content Creation Solutions for a Sports and Apparel Giant

- RESOURCES

- WHO WE ARE

What We Do Home
Generative AI
- AI Translation Services
- Content Remix
AI Training
- Aurora AI Studio™
Machine Translation
- MT Tracker
Instant Interpreter
Smart Onboarding
Translation Service Models
Content Services
- Technical Writing
- Training & eLearning
- Financial Reports
- Digital Marketing
- SEO & Content Optimization
Translation Services
- Video Localization
- Software Localization
- Website Localization
- Translation for Regulated Companies
- Interpretation
- Instant Interpreter
- Live Events
- Language Quality Services
Testing Services
- Functional QA & Testing
- Compatibility Testing
- Interoperability Testing
- Performance Testing
- Accessibility Testing
- UX/CX Testing
Industries Home
Life Sciences Translations
- Pharmaceutical Translations
- Clinical Trial Translations
- Regulatory Translations
- Post-Approval Translations
- Corporate Pharma Translations
- Medical Device Language Services
- Validation and Clinical
- Regulatory Translations
- Post-Authorization Translations
- Corporate Medical Device Translations
Banking & Finance
Retail
Luxury
E-Commerce
Games
Automotive
Consumer Packaged Goods
Technology
Industrial Manufacturing
Legal Services
Travel & Hospitality
Insights
- Blog Posts
- Case Studies
- Whitepapers
- Solution Briefs
- Infographics
- eBooks
- Videos
Webinars
Lionbridge Knowledge Hubs
- Positive Patient Outcomes
- Modern Clinical Trial Solutions
- Patient Engagement
- AI Thought Leadership
SELECT LANGUAGE:
In 2014, the Clinical Trials Regulation (CTR) was published and radically changed the regulatory landscape for clinical trials in Europe. The year 2024 also marks the end of the three-year transition period from the Clinical Trials Directive (CTD), which was repealed on January 31, 2022. On January 31, 2025, the CTR takes full effect. Ongoing trials with a Last Patient Visit after January 30, 2025, are required to transition from EudraCT to the Clinical Trials Information System (CTIS). After this transition, trial sponsors will find that a clinical trial language strategy is vital under the CTR because clinical trials become larger and content volumes increase. Read more to understand the potential language challenges of the CTR and how AI might assist with them.
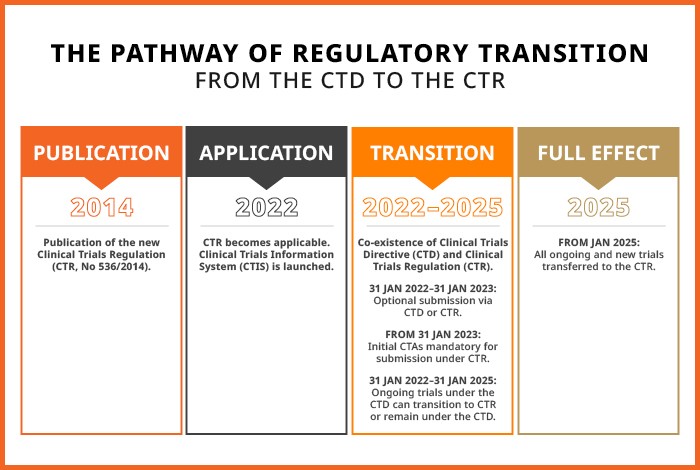
From Disruption to Excellence
The CTR is implemented to boost multinational clinical research in the EU. Despite the reform’s interim disruptions and burdens, the changes are implemented with a strong ambition to harmonize requirements and optimize clinical trial execution across the union. With the Clinical Trials Information System (CTIS) implementation, the clinical trials application procedure will become more efficient across the union. There will also be the possibility of a single submission and joint assessment across all concerned Member States. The new procedure reduces the standard 60 days to 45 — not counting extension periods for Request for Information.
Additionally, the legal transition from a Directive to a Regulation in the EU enables harmonization of technical requirements across Member States. This effect should make trial approvals and execution more efficient, thus benefiting sponsors, patients, and regulators. The public interface on the CTIS will also ensure more transparency of regional clinical research and give patients and potential trial participants access to information on regional clinical trials.
Purpose of the CTR as described by the European Medicines Agency:
“..foster innovation and research in the EU, facilitating the conduct of larger clinical trials in multiple EU Member States/EEA countries.”
Benefits of the CTIS as described by the European Medicines Agency:
- “Enables sponsors to apply for clinical trial authorisation in up to 30 European countries with a single online application;
- Allows national regulators to collaboratively process clinical trial applications in more than one country, request further information, authorise or refuse a trial and oversee an authorised trial;
- Facilitates the expansion of trials to other EEA countries;
- Enables transparency and access to information for any party interested in clinical trials conducted in the EEA through a searchable public website.”
An Average of Six Member States Across Multinational Trials
Since the 2022 CTIS launch, 3,657 clinical trial applications were submitted. In January 2024 alone, 309 Clinical Trial Applications (CTAs) were submitted. Out of these, 170 were initial CTAs, with commercial clinical trials having the highest proportion of multinational trials. 1,144 multinational trials had a decision in CTIS by January 2024; the average number of Member States in these trials was six. All of these trials would need translation of submission dossiers for content required in local official languages of EU Member States. The distribution of initial CTAs for multinational trials submitted since January 2022 is depicted below.

AI Solutions: Expansion and Speed Call for AI-powered Language Strategies
If the CTR successfully attracts larger clinical trials to the European Union, language will increasingly burden clinical trial budgets and clinical trial staff who manage clinical trial applications and In-Country-Reviews (ICRs). Additionally, language will require careful planning during trial preparation to ensure that all languages are ready for:
- Simultaneous submission via the CTIS
- Any updates during the short regulatory review windows
The system is based on a tacit decision principle. This means that if a sponsor does not respond within the deadline of a Request for Information from Member State authorities, the application will lapse for all concerned Member States.
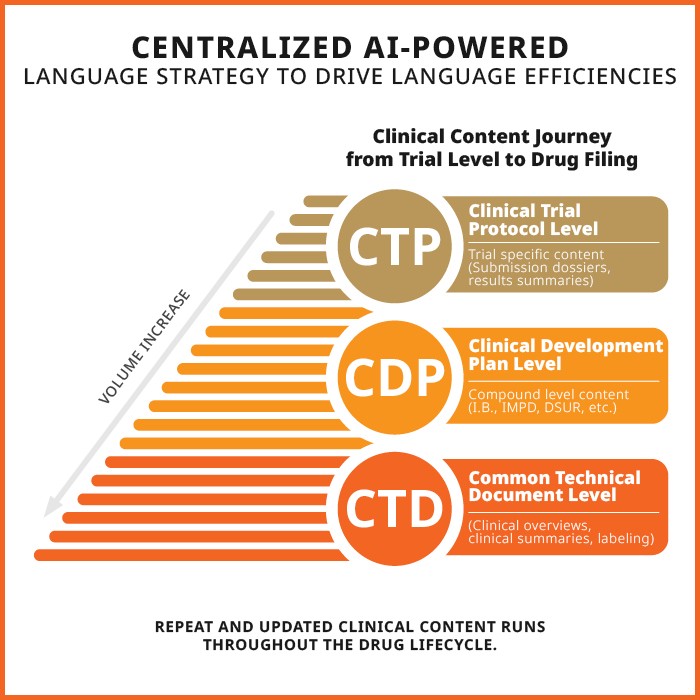
Large Language Models (LLMs) and Life Sciences AI services have potential to significantly speed up translations for clinical trials while also ensuring language consistency across large content volumes. Notably, LLM outcome quality is rapidly improving. Trial sponsors can benefit from AI solutions when combining strong language strategy with LLMs with predefined levels of human intervention. (They’ll also still reduce risk.) Such strategies can be designed at more levels, as suggested below.
Get in touch
Need assistance developing strategy for your life sciences translation? Curious about AI solutions for Life Sciences? Lionbridge has decades of deep knowledge and expertise in clinical trial translation. Contact us today to find out more about Lionbridge as a life sciences language service provider.



