AI Solutions
Additional Services

Stranger Danger: How Well Do You Know Your AI?
Global street smarts through culturalization.


Case Study: Multilingual Retail Marketing
New AI Content Creation Solutions for a Sports and Apparel Giant

- RESOURCES


In a prior blog, we covered the multilingual EU market and how the multilingual policy of the union impacts life sciences regulatory compliance regarding language requirements for regulatory translations. This blog will delve into the three levels of language requirements that these groups must know:
- Device manufacturer
- Economic operator
- Trial sponsor for EU medical products
Determining Regulatory Translation Needs in the EU
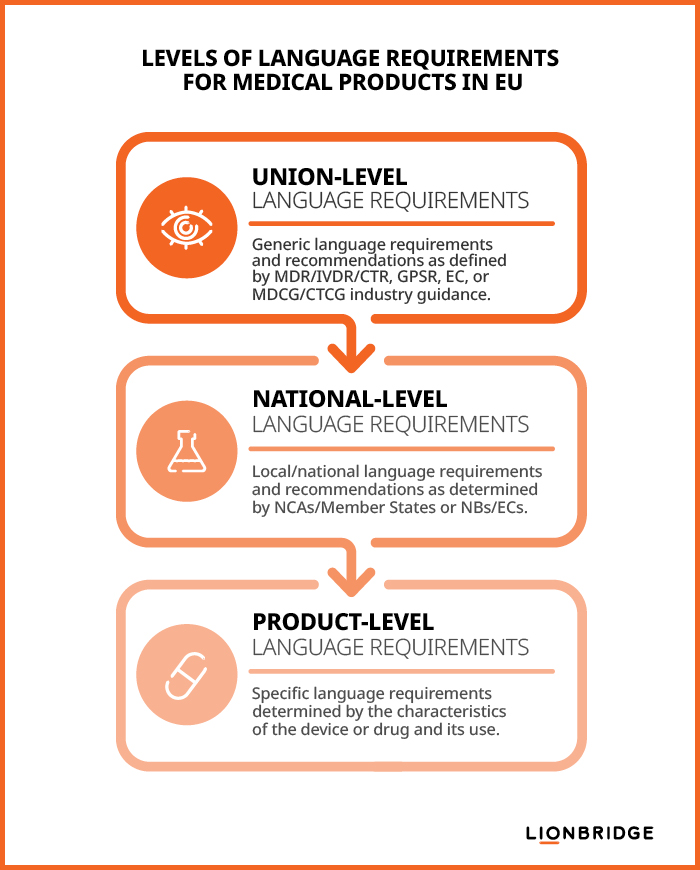
Under the EU Medical Device and Clinical Trial Regulations, a manufacturer or clinical trial sponsor should consider these three levels of language requirements to ensure life sciences regulatory compliance:
Union-level requirements: Defined in the MDR/IVDR/CTR, the General Product Safety Regulation (GPSR), European Commission (EC) publications, and industry guidance documents published by the Medical Device Coordination Group (MDCG) or the Clinical Trials Coordination Group (CTCG)
National-level requirements: Determined by each Member State’s National Competent Authorities (NCAs) and partly enforced by Notified Bodies (NBs) as part of conformity assessment procedures for medical devices or by Ethics Committees (ECs) as part of clinical trial authorization procedures.
Product-level requirements: Determined by specific product considerations, such as usage context, product characteristics, or intended user(s) or purpose(s).
Union-Level Language Requirements for Life Sciences Regulatory Compliance
Language requirements under the union level are general. Essentially, all consumer products placed in the EU are subject to the General Product Safety Regulation (GPSR). The GPSR requires these items to be provided in official language(s) as determined by the Member States where the products are made available:
- User instructions
- Labeling information
- Documentation for consumer products
For products subject to additional safety and performance requirements, such as medical devices and medicinal products, language requirements extend beyond the label and information supplied with the product. They apply, for example, when the manufacturer is responsible for ensuring concise, plain, or local language to support the safety and performance of devices. Union-level language requirements don’t determine which specific national language(s) are required per individual Member States.
National-Level Language Requirements
National Competent Authorities (NCAs) determine which specific national language(s) are required for each type of information for devices or drugs in their respective markets. Generally, all content intended for patients must be available in local language(s) in each Member State(s) to ensure patient safety. However, Member State authorities also consider other aspects of communication, such as:
- Readers’ literacy levels
- Technical/medical knowledge
- Training
NCAs in the EU differ regarding whether English is considered a commonly understood language and whether content intended for professional users is acceptable in English. In some Member States, such as France, Italy, and more Eastern European Member States, including Bulgaria, Hungary, and Lithuania, this content must be in local language:
- Marketing
- Field safety notifications
- Certification
English is accepted as an exemption in a few other countries, such as the Northern European member states Denmark and Sweden.
Product-Level Language Requirements
Product-level requirements include unique use cases for certain devices or drugs, for example, an In Vitro Diagnostic device intended for self-testing or near-patient testing or a mobile application used to capture patient data.
Manufacturers should consider the specific context of device or drug usage to determine language needs in cases where union or national-level publications don’t sufficiently address it. When in doubt, our global regulatory solutions experts recommend consulting the Notified Body or the National Competent Authorities before placing a device or drug on the market or in clinical studies.
Language Drives Access, Safety and Transparency
Manufacturers and clinical trial sponsors must ensure life sciences regulatory compliance with language requirements to enter the EU market. However, language considerations are more than a tick-box exercise. Making information available in local EU languages is critical to making information accessible, readable, and transparent for all product users or trial volunteers. Additionally, high-quality translations and addressing cultural nuances help manufacturers expand their market presence and ensure their products are used safely.
Get in touch
Challenged by regulated language outcomes in the multilingual EU? Partner with Lionbridge for regulatory solutions in the EU. We have decades of experience with EU languages and EU Regulations for medical devices and medicinal drugs. Our regulatory solutions support all product stages with our life sciences translation and language services.