- WHAT WE DO
Additional Services

- Industries

Case Study: Multilingual Retail Marketing
New AI Content Creation Solutions for a Sports and Apparel Giant

- RESOURCES

- WHO WE ARE

What We Do Home
Generative AI
- AI Translation Services
- Content Remix
AI Training
- Aurora AI Studio™
Machine Translation
- MT Tracker
Instant Interpreter
Smart Onboarding
Translation Service Models
Content Services
- Technical Writing
- Training & eLearning
- Financial Reports
- Digital Marketing
- SEO & Content Optimization
Translation Services
- Video Localization
- Software Localization
- Website Localization
- Translation for Regulated Companies
- Interpretation
- Instant Interpreter
- Live Events
- Language Quality Services
Testing Services
- Functional QA & Testing
- Compatibility Testing
- Interoperability Testing
- Performance Testing
- Accessibility Testing
- UX/CX Testing
Industries Home
Life Sciences Translations
- Pharmaceutical Translations
- Clinical Trial Translations
- Regulatory Translations
- Post-Approval Translations
- Corporate Pharma Translations
- Medical Device Language Services
- Validation and Clinical
- Regulatory Translations
- Post-Authorization Translations
- Corporate Medical Device Translations
Banking & Finance
Retail
Luxury
E-Commerce
Games
Automotive
Consumer Packaged Goods
Technology
Industrial Manufacturing
Legal Services
Travel & Hospitality
Insights
- Blog Posts
- Case Studies
- Whitepapers
- Solution Briefs
- Infographics
- eBooks
- Videos
Webinars
Lionbridge Knowledge Hubs
- Positive Patient Outcomes
- Modern Clinical Trial Solutions
- Patient Engagement
- AI Thought Leadership
SELECT LANGUAGE:
Patient-reported outcome (PRO) assessments have become valuable tools for incorporating patient voice in medical product development. PRO data can be collected throughout the full product life cycle, from early development to clinical trials and observational studies. PROs may be utilized as the basis for:
- Inclusion criteria
- Baseline assessment
- Efficacy assessment
- Safety assessment in clinical trials and/or post-authorization safety studies
Regulatory agencies, such as the U.S. FDA and EMA, fit PROs into the broad category of “patient experience data (PED).” PED reflect patients’ experiences, perspectives, needs, and priorities throughout their patient journey. These agencies used their strategic initiatives and guidance documents to promote PED as a key contributing component in product development and regulatory decision-making.
PED in Regulatory Decision-Making
However, a complete alignment between regulators and the industry on usage of PED is a work in progress. Despite the FDA’s strong advocacy for PED, it was reported that marketing authorization applicants, patients, and caregivers have insufficient clarity regarding how the agency uses such data in its decisions when reviewing applications for new drugs and biologics. Similarly, a multidisciplinary workshop led by the EMA revealed the industry stakeholders’ perception that the EU regulator doesn’t welcome PED or consider these data valuable.
These factors lead to industry stakeholders’ reluctance to include PED in support of marketing authorization applications. The EMA has since clarified that they “welcome and want” PED as part of marketing authorization applications.
To enable the generation of relevant, sufficient, and reliable PED, more transparency is required surrounding usage of such data in regulatory decision-making. Achieving this transparency requires a greater harmonization across jurisdictions of regulatory requirements for PED collected in multi-regional trials. As the European Federation of Pharmaceutical Industries and Associations (EFPIA) proposed, the ICH M4E presents the appropriate level for a global alignment on these requirements.

Better Tactics for Patient-Reported Outcome Data Collection
While essential to medical product development overall, regulatory uncertainties won’t likely be of immediate concern for trial participants. A more pressing issue is respondent burden, which application of PROs may give rise to. These various factors may contribute:
- Number of PRO questionnaires in a trial
- Frequency of PRO assessments
- Number of items in a PRO questionnaire/completion time
- Repetitive or similar questions across the PRO questionnaires used in the trial
- Unclear language
- Inconvenient format or mode of PRO data collection.
Anticipating and mitigating respondent burden is critical to overall success of PRO data collection strategy in a trial. Aiyegbusi and colleagues presented a succinct summary of key methodological considerations for addressing respondent burden. Among these are:
- Clear rationale for the selection of PROs for trial usage
- Schedule of assessments considerate of the nature of the condition and effects of trial interventions
- Support with completion of PROs via technology, where it would be appropriate
- Early patient involvement and engagement in decisions around selection and delivery of PROs

How Language Service Providers Help Improve Patient-Reported Outcome Data Collection
With the proliferation of multinational clinical trials and an increase in usage of ePRO technology, trial sponsors often have to modify original PROs. Modification may include translation and cross-cultural adaptation into other languages (linguistic validation) or migration from paper to the electronic mode of administration (ePRO migration). Testing modified versions with target patient populations may help identify and resolve issues before these versions are used in a trial. The benefits are manifold:
- Providing opportunity for patient involvement and engagement in clinical research and decisions around PROs
- Contributing to robust and reliable PRO data collected in the trial via “fine-tuning” PRO assessments through patient input
- Reducing burden for trial participants
- Meeting evidentiary expectations set by regulators regarding the process for modification of PROs for populations that will use them in the trial. This is particularly relevant where PRO data will be used to support product labeling claims.
Specialist language service providers (LSPs) may facilitate such testing through cognitive debriefing and usability testing procedures. Testing is conducted in relevant target country(-ies) with a small group of native speakers of target language/s who adequately represent the clinical trial’s target population.
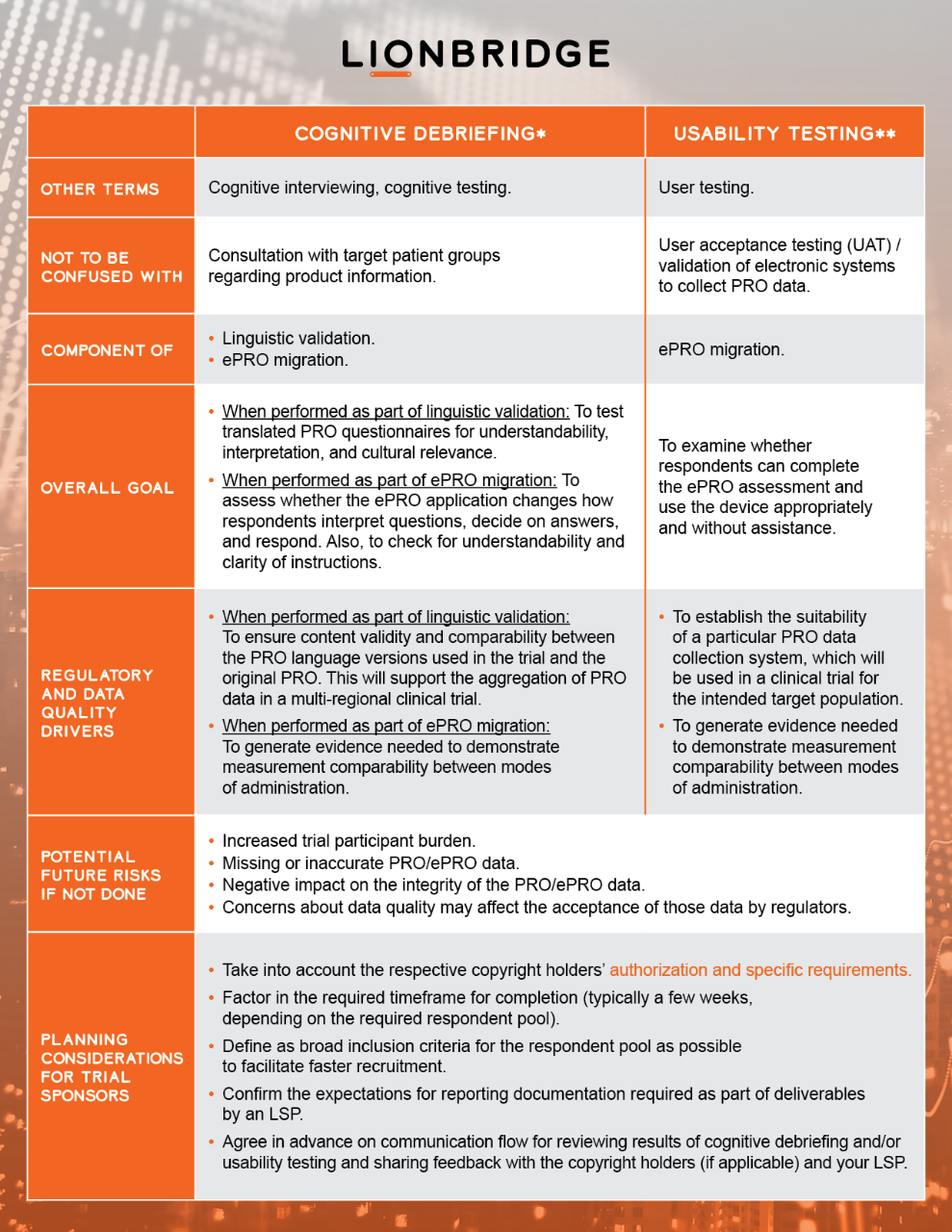
Copyright Permissions for Use of Clinical Outcome Assessments in Clinical Trials Blog
* with reference to ISPOR Task Force for Translation and Cultural Adaptation and Patient-Reported Outcome (PRO) Consortium
**with reference to ISPOR ePRO Good Research Practices Task Force and ISPOR Measurement Comparability Between Modes of Administration of PROMs Task Force
Get in touch
Need language assistance for your patient-reported outcome data collection in clinical trials? Lionbridge has deep knowledge of the clinical outcome assessment domain and expertise in life sciences translation services. Contact us today to learn more about Lionbridge as a life sciences language service provider.




