AI Solutions
Additional Services

Stranger Danger: How Well Do You Know Your AI?
Global street smarts through culturalization.


Case Study: Multilingual Retail Marketing
New AI Content Creation Solutions for a Sports and Apparel Giant

- RESOURCES


YPrime is an eClinical technology services company supporting eCOA and IRT. Over four years, it has grown from a tablet-based solution to one that is site-, web- and patient-based. The recent disruption in the life sciences field increased the pressure to deliver these services in a remote fashion around the globe.
Lionbridge Life Sciences expert Dan Herron spoke with Paulo Limgenco from the YPrime project management group and Chase Carmeans, their Senior Portfolio Director, about how their team responded to the pandemic and how YPrime applies multilingual translation to expand their services.
Dan: How has COVID-19 impacted your business?
Chase: When patients aren’t visiting sites, they can’t get the handheld devices they would usually use to respond to trial questions. The pandemic also made staff at study sites understandably uncomfortable using devices that haven’t been disinfected. So we rolled out our web backup solution, historically and strictly site-based, to patients as well. We also made it possible for sites to activate this alternative themselves, removing YPrime from a privacy perspective.
Our tool emulates eCOA software exactly: you’ll see an actual image of the tablet that would have been handed to you pre-pandemic. There was no proven electronic backup methodology in this industry--previously trial sites would simply revert to paper if an electronic device broke. But paper is kind of a four-letter word in our space. We saw this as a need our tools could fill, even before travel restrictions and quarantine orders were in place.
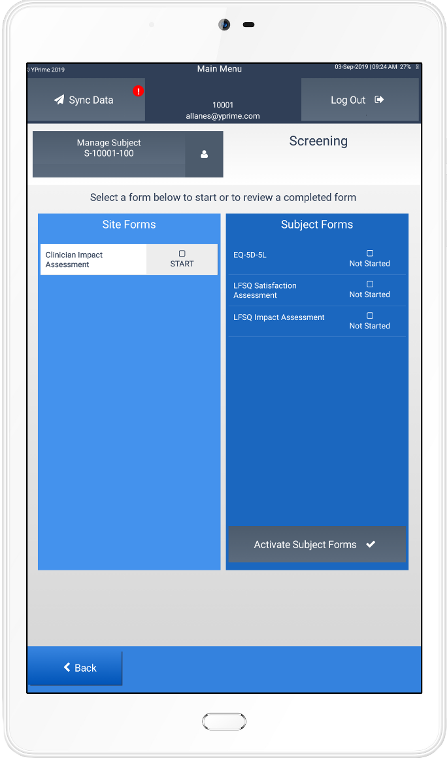
Paulo: We are usually a more proactive than reactive partner in study execution, but the health crisis has forced everyone to respond in real time. Our web backup solution was not originally intended for this exact purpose. Thanks to the creativity of this team, we were able to quickly repurpose this solution to meet the current needs of the business.
DH: How do you differentiate yourself from your competitors?
CC: We have an app-based solution, which means if trial runners have a dual modality, e.g. tablet and handheld, we build the application once to work on any device.
Our solution is also highly configurable. When we are building a study, it’s in a prevalidated state. We do write custom code when we need to, but when we kick a study off it’s already 80 percent configured. Our competitors need to rewrite far more than we do, which adds risk, drags out timelines and increase costs for their services. YPrime, on the other hand, can show up at kickoff meeting, with a demonstration because we can spin these things up really quickly. Ultimately, we’d want to get to a place where we’re 100 percent configured.
DH: What challenges was YPrime facing when you realized you needed a language services provider?
CC: We realized we needed an LSP almost immediately. Nearly every study we work on has at least one additional language. A relatively simple US-based study will use English and Spanish, but we work with the top ten of pharma, on phase three global studies, so we could be talking about 20 to 40 languages per study.
Timelines, and transparency around them, are always huge with us. How quickly can we provide translations? What are our deliverables? It’s important to us that we have deliverables and a relationship with project managers.
DH: I remember us completing an audit and YPrime coming on site to set up the right workflows. How else did Lionbridge address your challenges? What makes the relationship work?
PL: From our perspective, working with Lionbridge means we’ve been able to do what we say and say what we do. With some previous partnerships, quality or communications were falling short, and we were left to answer as the middleman. I have not seen this happen in our work with Lionbridge.
Aside from sticking to timelines of the work we’re doing together, and maintaining transparency and communications, one of the great things I’ve experienced with Lionbridge is that it’s truly a relationship. Sometimes these types of partnerships can feel like a means to an end, but in this case the synergy between these orgs has been clear from the get-go.



